Oxygen Signals and Paleoclimates
(Reading #7 for my course on Climate Change, Alan Holyoak, PhD)
Note: This is the last reading in this series. This set of 7 readings is designed to help college general education students gain the the foundational background they need to understand the contents of the book "The Climate Crisis" by Archer and Rahmstorf, which I use as a course textbook.
Daily Objectives
1. Be
able describe the differences between heavy and light oxygen isotopes.
2. Be
able to explain why ice cores from polar glaciers contain meaningful climate
records.
3. Be
able to explain why speleotherms, wood, and coral contain meaningful climate
records.
4. Be
able to explain why paleoclimatologists probably get excited when they find
skeletons of foraminiferans in their sediment samples.
5. Be
able to comment on the general pattern of global temperature change over
billions, millions, and hundreds of thousands of years.
Introduction
A
way tool to gain insight into current patterns of climate change is to learn as
much as possible about Earth’s climate history.
This field of study is called paleoclimatology. Paleoclimatologists collect data from as
many sources as possible to help them develop a picture of what Earth’s climate
was like in the past. These observations
range from glacial erratics and glacial striations that you have already
learned about to oxygen and carbon isotope ratios in ice, water, and sediments,
among other things. In this reading you
will learn about the data that paleoclimatologists collect to investigate Earth’s
climate history. One important thing to
keep in mind is that the farther we look back in time, the greater the range of
uncertainty there is in the data.
Climate Clues
Just
like a detective, paleoclimatologists are good observers and creative thinkers. This helps them identify and make sense of
the climate clues that exist in Earth’s historical record. You already learned how boulders and rocks
that seemed out of place and strange scratches on rock faces led to Louis
Aggasiz’s 1837 Ice Age Theory.
Similarly, other good observers discovered other ways to tease more clues
about Earth’s climate history from sources such as ice, wood, ocean sediments,
and even stalagmites.
Climate and oxygen isotopes
All
isotopes of an element have the same
number of protons, but different numbers of neutrons. This difference in neutron number gives each
isotope a unique atomic mass. There are
three isotopes of oxygen, 16O, 17O, and 18O. These isotopes are not radioactive so their global
concentrations are stable. Their
characteristics are listed in Table 1.
Table 1. Characteristics of isotopes of oxygen
Oxygen Isotope
|
Number of Protons
|
Number of Neutrons
|
Proportion of all oxygen atoms
|
Oxygen-16 (16O)
|
8
|
8
|
99.76%
|
Oxygen-17 (17O)
|
8
|
9
|
0.04%
|
Oxygen-18 (18O)
|
8
|
10
|
0.20%
|
Paleoclimatologists
are interested in finding light oxygen
(16O), and heavy oxygen (18O) in
compounds in samples they collect. They
use the ratio between 18O and 16O atoms in
oxygen-containing compounds as proxy data for temperatures of past time
periods. Fortunately these kinds of
compounds are found in ice, sediments, fossils, and other long-lived
substances. The heavy to light oxygen ratio
is calibrated against a standard of heavy to light oxygen isotopes in seawater 200-500
meters deep in our ocean today. The
ratio between light and heavy oxygen-containing compounds at this depth correlates
extremely well with average surface seawater temperature. It therefore stands to reason that whenever
we find oxygen ratios similar to those in today’ oceans and associated seawater
temperatures, the same oxygen-ratio/seawater temperature relationship should
have existed in oceans throughout history.
The
vast majority of water molecules are made with light oxygen, but the rest
contain heavy oxygen (Table 1). Scientists
discovered that the rates of evaporation and condensation of water with high
and heavy oxygen are not identical; water with light oxygen evaporates slightly
more readily than water with heavy oxygen, and water with heavy oxygen tends to
condense and fall as precipitation before most of the water vapor that is made
of light oxygen (Fig. 1).
Figure 1. Relationship between temperature and heavy oxygen (18O)
concentration in precipitation. These
data show the percent divergence from the standard 16O:18O
ratio in the ocean. Negative values mean
there is less 18O present than in the ocean standard. (Image: NASA)
When
air cools water vapor condenses and falls as precipitation. 18O has
a greater mass than 16O and water made with heavy oxygen therefore has
a greater mass, and as subject to a greater gravitational force than something
that has a lower mass. As a result,
water made with 18O falls more readily than water made with 16O. Water vapor left in the atmosphere at this
point is partially 18O-depleted.
This depletion happens faster when temperatures are low than when they
are high. Therefore, when the Earth is
in a cool phase most of the 18O-water precipitates out before water
vapor reaches the poles, leaving mostly 16O water to fall as snow to
form ice layers there. Conversely, when
the Earth is in a warm phase 18O-water stays in the air longer, and
more 18O reaches the poles than when the Earth is cool. This produces a 16O to 18O
ratio in polar ice that contains elevated levels of 18O when the
Earth is warm (Fig. 2). Scientists have
measured the ratios of oxygen isotopes in ice layers from polar ice caps to
produce accurate records of climate change going back as far as the ice record. These data currently go back 800,000 years. Fortunately,
we can look even farther back using other kinds of data, because oxygen isotope
ratios go only so far back using ice alone.
Figure 2. Water rich in both heavy and light oxygen evaporates at
the equator, but as air moves away from the equator it cools, and heavy oxygen
water falls as rain at a faster rate than light oxygen water. During an ice age polar ice there will be
significantly less 18O in the ice than when the Earth is in a warm
phase. (Image: NASA)
Before we look
at other things that contain oxygen ratio data, however, there is something
else of interest that oxygen ratios in water can tell us. These ratios can tell us whether a particular
time period was wet or dry. This is the
case because most of the heavy oxygen tends to condense first and fall as
precipitation over oceans, leaving mostly light oxygen water to move onshore to
fall as rain over continents. So, if
sediments in a region of ocean have increased amounts of 16O in it,
this is almost certainly the result of freshwater runoff from continents. When this is observed we conclude that Earth
was experiencing a wet climate.
Diversity of Oxygen Records
Ice was pointed
out in the previous section as an extremely important source of climate
data. Why is this the case? Scientists drill cores of ice from polar
glaciers (e.g., Antarctica and Greenland) and collect date-specific data from
water and other materials trapped in ice (Fig. 3). The cores from this drilling show distinctive
layers that are produced annually (Fig. 4 & 5). Scientists take samples from each layer,
analyze the ice for oxygen isotope ratios and other materials trapped in the
ice, and can thereby determine the temperature of the Earth when the ice was
formed.
Figure 4. This photograph was taken when scientists in Antarctica
dug a trench, but left a thin wall of snow between the two halves of the trench. Light illuminating the wall clearly shows the
annual layering of snow, which eventually gets compacted into ice layers. The stuffed animal is included for scale. (Image courtesy of NASA.)
Figure 5. These images show ice cores from different depths within
a polar ice cap. The upper image clearly shows layers of ice in the exposed side of an ice sheet. The lower image shows layers of ice from ice
cores, and that they can be quite distinctive, depending on their depth and
age, as well as anything else that is trapped in the ice. (Images: NASA.)
Oxygen
isotopes are found in more materials than just ice. It is also stored in wood, shells, bone,
coral, and some kinds of rocks. One
extremely important source of temperature data is stored in the microscopic
skeletons of tiny organisms called foraminiferans. These small organisms are related to amoeba,
but they produce calcium carbonate
(CaCO3) shells. The shell of
each species of foraminifera has its own unique shape and sculpturing (Fig. 6). This means that whenever a scientist spots
foraminiferan shells, they can know what species of foraminiferan they are
looking at, and whether those foraminifera were warm-water or cold-water
species. Looking for the presence of warm
and cold-water species, and the oxygen isotope ratios in their shells in sediment
layers provide important clues about paleoclimates. This is one of the reasons why climatologists
drill sediment cores as well as ice cores (Fig. 7).
Figure 7. Drilling ships like the one above are used to collect sediment
cores for analysis. Scientists slice the
sediment cores, do chemical analyses of each layer, and look for foraminifera
and other evidence of climate change. (Image: NASA.)
Paleoclimate
records also exist in stalagmites
that are formed in caves and caverns (Fig. 8).
These kinds of climate data are called speleotherms. How can speleotherms
contain meaningful climate information? Structures
in caves are largely isolated from the surface and do not experience processes
of erosion like rocks on the surface do, so once materials are deposited there
they remain there indefinitely.
Stalagmites are
produced by water that trickles through the soil and rock layers above the
cave. As water moves through the soil
some materials, like CaCO3 dissolve in it. When water drips into caves and then
evaporates, the CaCO3 is left behind. A new layer of CaCO3 is added to a
stalagmite each year, so if you slice through a stalagmite and polish the cut
edge the individual layers are visible (Fig. 9). Just like tree rings, the rings in
speleotherms are wider during wet years and narrower during dry years, and
samples can be taken from each layer for analysis. But, how can we know the ages of the layers of
a stalagmite? Again, think back to FDSCI
101 and your discussions on radiometric
dating. That works here too!
As
indicated in the text below Fig. 9, Uranium-Thorium radiometric dating can be
carried out on a sample from each layer of a speleotherm. Uranium readily dissolves in water and is
deposited along with CaCO3 then the water evaporates. Thorium, however, does not dissolve as
readily in water, so all Thorium in a layer is the result of Uranium decay, and
can be used to calculate an accurate age for each layer. Pretty slick, huh?
Figure 9. This is
a photograph of a cross-section cut through a small stalagmite. Each band represents one year of deposition
of chemicals from water dripping from above.
Samples from each section can be used to measure oxygen isotope ratios
and to carry out radiometric dating, as indicated in the text below the
photo. (Image courtesy of NASA.)
Climate
is, of course, more than just temperature.
It is also includes the pattern of precipitation area experiences. Figure 10 shows the relative precipitation
record for the region around Carlsbad Cavern, NM, based on speleotherm data.
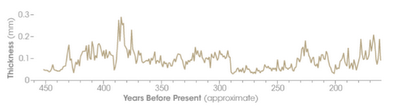
Figure 10. A record of
stalagmite ring thicknesses from Carlsbad Caverns, NM. The ring thickness provides a record of
relative amounts of rainfall in that area over the past 450 years. It is
typical for any natural system to exhibit variability around the overall trend.
(Image courtesy of NASA.)
Scientists
can also use tree core data to decipher recent climate changes. As mentioned about in relation to
speleotherms, trees in temperate and Polar Regions produce a pair of rings of
wood each year. In years when conditions
promote abundant growth trees produce a thick tree ring. In years when
conditions limit growth trees produce a narrow ring (Fig. 11). By producing overlapping tree ring records
scientists have produced climate records extending back thousands of years. Bristlecone pines are among the known longest
living trees, and a climate record using their tree rings has been produced
that goes back over 5,000 years (Fig. 12).
Figure 11. This photograph shows part of the cross section of a
trunk from a tree. The tree rings vary
in width depending on local climate conditions; wide rings indicate favorable
growth years, and narrow rings indicate years in which growth was limited.
(Image: NASA)
Figure 12. Precipitation record from overlapping Bristlecone Pine
tree ring data (blue line) compared to the average precipitation from the 20th
Century (tan line). Scientists apply
knowledge about the relationship between precipitation, temperature, soil
quality, and other factors and tree ring thicknesses in living trees to reach
conclusions about past climate conditions.
(Image: NASA)
While
speleotherms and tree rings are useful for reconstructing terrestrial climate
conditions, they do not help us understand how climate changes affect
conditions in the ocean. Fortunately,
while trees produce annual tree rings, corals
also produce records of annual growth that is recorded in their CaCO3
(Fig. 13). The CaCO3 secreted
by corals occurs in the upper levels of the ocean, so we can analyze each layer
of coral skeletons to discover the 16O and 18O ratios to see
how climates changed in the tropics during recent history (Fig. 14).
Figure 13. This x-ray of a cross section through a coral colony’s
skeleton exposes the annual layers of growth.
The heavy and light oxygen ratios in the CaCO3 that makes up
each layer provides a signal that can be used to generate a climate record
extending back as far as the age of the colony.
Some colonies are known to have lived well over 1000 years. (Image
courtesy of NASA.)
Figure 14. Oxygen isotope
ratios were used to generate a temperature profile for ~1890-1950 (lower
graph). These data are compared to
periodic ENSO (El Nino Southern Oscillation) oscillations that occur in the
tropical Pacific Ocean to see if correlations exist (upper graph). Red areas represent temperatures above the
long-term average, and blue areas show cooler temperatures. Dark gray vertical bars represent strong El
Nino events, and light gray bars represent weak El Nino events. (Image: NASA.)
Climate Patterns in Deep Time
Paleoclimatologists
use data from sediment cores, ice cores, speleotherms, tree rings, coral growth
layers, and other data, to reconstruct Earth’s climate history. Several groups of climatologists, working
independently, generated climate models using available data to provide
estimates of past climates throughout Earth’s history. While these models represent the best science
on this topic, the farther we go back in time, the less precise the scenarios tend
to be. Earth history with only a relative indication of temperature
extremes. The reconstruction of Earth’s
climate history is shown in Fig. 15. This
figure is based on sea level change, proxy data of CO2
concentrations in the atmosphere, and other proxy data from the geologic
record. Figure 16 shows a model of CO2
levels from 400 Ma (million years ago) compared to proxy temperature data.
Figure 15. This
figure shows a deep time reconstruction of Earth climate history. Earth has oscillated between cold and warm
periods throughout its history. Cold
periods are indicated in blue and warm periods in…peach J? Observe that the time
scale on the left side of the figure is not linear. It is roughly logarithmic, with older time
periods being allocated less space than recent time periods. This is appropriate since we have much better
data for recent time periods than for more ancient times, but if one is unaware
of this it can give someone a skewed view of earth’s climate history. (Image from WW Norton, adapted from Kump et al. 1999.)
Figure 16. Model of paleo-CO2 levels, comparing the model to other predictors of climate. (Image from Archer and Rahmstorf.)
Climate
reconstructions from deep geologic time are helpful in showing general trends,
but if we hope to identify current climate trends and make sense of what is
happening now, we must compare what is happening today with more recent climate
histories. For example, by using oxygen
isotope data from ocean sediments we can generate a relatively precise record
of climate from 65 Ma. This
reconstruction is shown in Fig. 17. Many
climatologists who have studied the Paleocene-Eocene Thermal Maximum (PETM)
have suggested that evidence of events before and during this thermal maximum
around 55 Ma are similar in some respects to trends we are experiencing today.
Figure 17. Reconstruction of past climate using evidence from the
deep ocean, including oxygen isotope ratios and other evidence from sediment
cores. (Image from Archer and Rahmstorf.)
As
shown in Fig. 17, Earth has, with a few ups and downs in global temperature,
undergone gradual cooling over the past 65Ma.
As we look at progressively shorter time intervals we see that over the 400,000
years that Earth has experienced a temperature equilibrium including
oscillations between cool and warm periods, i.e., glacial and interglacial
periods.
Figure 18. The past 400,000 years of Earth’s temperature
variability. Four prolonged glacial
periods are indicated in blue, and warm interglacial periods are shown in red.
(Image adapted by ARH from a figure courtesy of NASA.)
The
bottom line when it comes to Earth’s climate history is that climate has
changed in the past, it’s changing today, and it will continue to change in the
future. This conclusion is evidenced by
the fact that we have seen periods of Earth’s history where it was probably a
lot like a giant ice ball, and other times when it was like a giant hot
house. Geologically speaking, recently Earth
has experienced a more or less stable climate cycle of warm and cool
periods. So, when we begin to consider
the current state of the climate, this recent trend is the most meaningful
baseline we have for comparison to what we see today. Deviations from Earth’s recent past stable
climate conditions deserve to be investigated.
Those are, of course, topics for other class discussions, and we will
address them later in the semester.
Source material
Archer, D., and S. Rahmstorf. 2010. The Climate Crisis.
Cambridge University Press.
Riebeck, H. 2005. Paleoclimatology. NASA Earth Observatory
Program. http://earthobservatory.nasa.gov/Features/Paleoclimatology/



















No comments:
Post a Comment